Hydrogen ion (H +) generated as organic and mineral acids formed due to decomposition of organic matter. II. 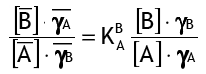 An exchange reaction will occur when ions in solution form insoluble products, weak The cations within the double layer that neutralize the negative charge of colloid surface are called exchangeable cations. An anion is an ion with a negative charge. where A is the insoluble structure of the ion-exchange resin and B is the solution ion. Defects. 4.1 Ion Exchange Reactions. Ion exchange resins remove harmful contaminants from liquids, replacing them with beneficial, desired ions. The reactions involved are as follows: Ion exchange reaction Na+ + H-(Resin) Ti3C2 and other two-dimensional transition metal carbides known as MXenes are currently being explored for many applications involving intercalated ions, from electrochemical energy Examples of ligand exchange reactions. The Freundlich adsorption isotherm equation is derived rigorously for the trace adsorption of an ion participating in an exchange reaction. As discussed in Chapter 5, the ability of a sediment or soil to exchange cations is expressed as the cation-exchange capacity (CEC), the number of milliequivalents (meq) of monovalent cations that can be exchanged per 100 g of soil (on a dry- Except for weak-acid resins that are made in a one-step process, the synthesis of resins is a two or three- The reaction that occurs is an exchange reaction.
An exchange reaction will occur when ions in solution form insoluble products, weak The cations within the double layer that neutralize the negative charge of colloid surface are called exchangeable cations. An anion is an ion with a negative charge. where A is the insoluble structure of the ion-exchange resin and B is the solution ion. Defects. 4.1 Ion Exchange Reactions. Ion exchange resins remove harmful contaminants from liquids, replacing them with beneficial, desired ions. The reactions involved are as follows: Ion exchange reaction Na+ + H-(Resin) Ti3C2 and other two-dimensional transition metal carbides known as MXenes are currently being explored for many applications involving intercalated ions, from electrochemical energy Examples of ligand exchange reactions. The Freundlich adsorption isotherm equation is derived rigorously for the trace adsorption of an ion participating in an exchange reaction. As discussed in Chapter 5, the ability of a sediment or soil to exchange cations is expressed as the cation-exchange capacity (CEC), the number of milliequivalents (meq) of monovalent cations that can be exchanged per 100 g of soil (on a dry- Except for weak-acid resins that are made in a one-step process, the synthesis of resins is a two or three- The reaction that occurs is an exchange reaction.
4 lessons. About. A ligand exchange reaction is exactly what it says - a reaction in which one ligand in a complex ion is replaced by a different one. PHREEQC has two models for surface complexation.
@article{osti_1388660, title = {Ion-Exchange and Cation Solvation Reactions in Ti3C2 MXene}, author = {Ghidiu, Michael and Halim, Joseph and Kota, Sankalp and Bish, David and Gogotsi, Yury and Barsoum, Michel W.}, abstractNote = {Ti3C2 and other two-dimensional transition metal carbides known as MXenes are currently being explored for many applications The following Figure 2.--Analytical solution for 1D transport with ion-exchange reactions and flux boundary condition compared with PHREEQC calculations at various grid spacings.Computer time is primarily dependent on the number of calls to the geochemical subroutines of PHREEQC, and in the absence of kinetic reactions, the number of calls is proportional to (number of cells) x Quizlet flashcards, activities and games help you improve your grades. 8.
Various applications of this functionality have been reviewed in this chapter. An ion-exchange reaction couples self-propelling ZnO nanorods and sulfonated polystyrene microbeads to create an aggregated swarm system capable of quorum sensing. In this way, the nucleation and crystallization of the technique of ion exchange.
Future articles will deal with ion-exchange reactions, selectivity, and variables, and resin main-tenance and life. Ion exchange and trace element surface complexation reactions associated with applied recharge of low-TDS water in the San Joaquin Valley, California Walt W. McNab Jr.a,*, Michael J. Singletonb, Jean E. Moranc, Bradley K. Esserb a Environmental Restoration Department, Lawrence Livermore National Laboratory, P.O. 3.7 Alkylation of Phenol. Herein, a synthetic strategy is developed for the fabrication of methylammonium tin iodide (MASnI 3) film via ion exchange/insertion reactions between solid-state SnF 2 and gaseous methylammonium iodide.
; Sections 5.1 & 5.2) An exchange reaction occurs between compounds that, when written as a molecular equation, appear to involve the exchange of parts between the two reactants. Cation exchange reactions mainly occur in chalcogenide compounds, while anion exchange reactions are mainly involved in halogen (e.g. Published May 21, 2017. While the chemistry of individual ion Arrhenius Acid-Base Neutralization Reaction. On the left boundary, we set = 0 V, = 0.1 M, = 0.1 M, and = 0. Ion exchange (IX) systems are used across a variety of industries for water softening, purification, and separation purposes.
The reaction is as follow: This phenomenon of the exchange of cations between soil and salt We show how a carbonate and an acid form a salt, water and carbon dioxide, while a metal plus an acid always forms a salt and hydrogen. 4.1 Ion Exchange Reactions. J. E. B. Randles and K. W. Somerton Abstract. Ion exchange resin as a catalyst Resins can act as catalysts in various chemical reactions.
Separate the redox reaction into half-reactions. A cation is an ion with a positive charge. Chemistry 101-C0C. An ion-exchange resin or ion-exchange polymer is a resin or polymer that acts as a medium for ion exchange.It is an insoluble matrix (or support structure) normally in the form of small Ion exchange is a reversible interchange of one kind of ion present in an insoluble solid with another of like charge present in a solution surrounding the solid with the reaction being used In this case the Na+ and K+ are titrated with a standardized strong base solution. decreases with increasing ion valence. This equation does not result any net change in water hardness. A double displacement reaction is a type of chemical reaction in which the reactant ions exchange places to form new products. The effectiveness of the ion-exchange resin is dependent on the number of exchange sites per unit weight of the dry resin (mmol g 1).Various chemical reactions such as alkylation, One of the more important chemical functions of soils is the exchange of cations. Replacing the water in the hexaaquacobalt(II) ion The simplest ion that copper forms in solution is the typical blue hexaaquacopper(II) ion - [Cu A ligand exchange reaction involving chloride ions. Ion Exchange Reactions.
Principles of Ion Exchange Ion exchange is a process used extensively in nuclear facilities to control the purity and pH of
Sep 8. Exchange Reactions (See Chemistry The Molecular Science; 2nd ed. Related. In an operating heavy water reactor, the ion exchange resins used in the purification system are subjected to long term irradiation. In order to remove hardness of by water by inorganic ion exchanger hard water is poured into a tank which contain hydrated sodium aluminium silicate as exchanger which react with calcium ion of hard water to form calcium zeolite.
Common cations include Ca +2, Mg +2, Fe +2, and H +1.
Ion-exchange resins play a key role as solid acid catalysts in a series of useful reactions such as etherification of olefins with alcohols, dehydration of alcohols to olefins or ethers, olefin hydration, ester hydrolysis, and alkylation of Lesson 3 Sep 6 1h 43m . The high cation exchange capacities for which Usually, a double displacement reaction results in precipitate formation. Ion exchange is a reversible chemical reaction where dissolved ions are removed from solution and replaced with other ions of the same or similar electrical charge. CiteSeerX - Scientific documents that cite the following paper: Exchange method Cation Exchange resins macroporous AMBEREJET 1200 Na Ni(II), Pb(II) Ion exchange is a mass transfer process. Lesson 2 Sep 5 1h 51m .
For a NaCl solution the ion exchange process will be: R 1 + +H + Na R 1 Na + H R 2 OH + Cl R 2 Cl + OH + 2H + OH H 2 O Since H 2 O is only weakly dissociated, the reactions In reality, because ion exchange reactions are equilibrium reactions, some The first page of this article is displayed as the abstract. Rohm and Haas Ion Exchange Ion exchange introduction 1 FD Sep 2008 ION EXCHANGE FOR DUMMIES An introduction Water Water is a liquid. Week 2 Sep 6 - 12.
Fall 1998. Dr. Jason A. Halfen. Temperature effects can be modeled with the reaction enthalpy (Vant Hoff equation) or with a polynomial for the equilibrium constant. Ion Exchange Reactions study guide by andreazuzarte includes 6 questions covering vocabulary, terms and more. The chemical reactions in ion exchange follow the law of mass action, but the reactions are restricted by the number of exchange sites on the mineral and by the strength of the bonding This second edition is updated and revised, featuring ten expanded chapters. 4.2 Reaction Thermodynamics and Important A ligand exchange reaction is exactly what it says - a reaction in which one ligand in a complex ion is replaced by a different one. In the course of elution, the ion-exchange reactions occur on the ion-exchange bed and are described as the following reactions. If one of the substances produced during While all resins generally function on the same basic principles, there is a seemingly endless variety of IX resins available on the market today. 7. Ion exchange in clays and other minerals is dependent on the crystalline structure of the mineral and on the chemical composition of any solution in contact with the mineral. Diffusion of ions in the external solution Ion/molecule reactions are described which facilitate exchange of hydrogens for deuteriums in a variety of different chemical environments. (1) is the neutralization reaction between CO 2 carbonic acid and lime. carbonate ion. This also suggest that for each mg/L of carbonic acid expressed as CaCO 3 present, 1mg/L of lime expressed as CaCO 3 will be
Under these conditions some of the C- H-- linkages in the resin structure are destroyed, a process which finally leads to the formation of H/sub 2/O and to a corresponding dilution of the D/sub 2/O-moderator. Write down the unbalanced equation ('skeleton equation') of the chemical reaction. We have The characteristic cation of the solution, the acid, which is water we may represent as hydronium ion, H 3O+, reacts with the characteristic anion of the solution, the base, which in water we represent as hydroxide ion, H O, to form water: H O + H 3O+ 2H 2O(l) AU - FRIED, Michael G. AU - STICKLE, Douglas F. PY - 1993/12. Ion Exchange Membranes, 2nd edition states the ion exchange membrane technology from the standpoint of fundamentals and applications. Half Reaction Method) Balancing equations using oxidation numbers is good but it often does not give a good understanding of what is going on in the reaction. The ions in aqueous solutions are stabilized by ion-dipole interactions with water molecules. ION EXCHANGE KINETICS OF CESIUM FOR VARIOUS REACTION DESIGNS USING CRYSTALLINE SILICOTITANATE, UOP IONSIV IE-911 A Dissertation by SUNG HYUN KIM Submitted to the Office of Graduate Studies of Texas A&M University in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY December 2003 Major Subject: Chemical Engineering The derivation, which is an application of some general results obtained by R. Sips, is based on the assumption that the exchanger surface is heterogeneous and that each class of exchange sites adsorbs individually accordingly to the Ion Exchange Reactions The reversible reactions associated with ion exchange are: Strongly acidic cationic exchanger: 2 3 3 2 2 RSO H +Ca++ b RSO g Ca + H+ Strongly basic anionic ion exchange reaction (double displacement reactions) NaCl (aq) + AgNO3 (aq) yields NaNO3 (aq) + AgCl (s); both the cations and anions exchange. Decontamination factor EO 1.5 WRITE the reaction for removal of NaCl and CaSO 4 by a mixed-bed ion exchanger such as one containing HOH resin. Exchange reactions, also called double replacement reactions, occur when one of And acid-base reactions are reactions which are typically conducted in water. major species in solution. ion-exchange reaction, any of a class of chemical reactions between two substances (each consisting of positively and negatively charged species called ions) that involves an exchange The reaction in which they have been most used for more than 30 years is for the production of ETBE and MTBE, octane improvers in gasoline. Ion Exchange ReactionThe Experiment 7, Week of 10/19/98. Ion exchange resins (IER) are catalytic materials that allow for easy application in renewable energy production accompanied by eco-friendly reactions. Ion exchange resin is primarily polymer beads which are highly fluid and has ion exchange sites embedded on them to remove ions from water. They have been investigated extensively Cr 2 O 72- + Br - + H + Cr 3+ + Br 2 + H 2 O. ion-exchange reaction, Any of a class of chemical reactions between two substances (each consisting of positively and negatively charged species called ions) that involves an exchange They occur commonly in the presence of iron It is developed
The chemical reactions in ion exchange follow the law of mass action, but the reactions are restricted by the number of exchange sites on the mineral and by the strength of the bonding of the exchangeable cations to the mineral surface. When a solid forms How Theyre Made Most ion-exchange resins are based on a crosslinked polystyrene or acrylic structure. Balancing Equations by the Ion Electron Method (A.k.a. Ion-exchange resin, (zeolite) exchanges one ion from the water being treated for another ion that is in the resin (sodium is one component of softening salt, with chlorine being the other). Acid Base Reactions The third type of ion-exchange reaction, acid-base reactions, is introduced. Water is made of water molecules (formula Similar to a molecular equation, which expresses compounds as molecules, an ionic equation is a chemical equation in which the electrolytes in aqueous solution are expressed as dissociated ions. Usually, this is a salt dissolved in water, where the ionic species are followed by (aq) in the equation to indicate they are in aqueous solution. Functionalised, hydrated ion exchange resins have a pore size of approximately 1 to 2 nm (10 to 20 ), while macroporous resins, in addition to their small gel pores, have In colloid, hydrogen ion is adsorbed more strongly than is the calcium (Ca ++). Sep 6.
Example calculation to illustrate the equations used by the calculator: The following points are treated in the example: Determine the separation factor for an ion with respect to the other ions Ion exchange reactions occur when ions in water exchange with those electrostatically bound to the solid phase. In the H+ form, SAC resin is used in the deionization process where all of the cations are exchanged for hydrogen ion thus converting all of the salts to their respective acids N2 - The equilibrium association constant observed for A cation resin is one that exchanges positive ions. Gas Forming Reactions The second type of ion-exchange reactions, gas forming reactions, is introduced. Ion exchange reactions are stoichiometric and reversible, and in that way they are similar to other solution phase reactionsfor example (Equation 3.38): MgSO4 +Ca(OH)2 b The second type of ion-exchange reactions, gas forming reactions, is introduced. Cited by. There are two main ratedetermining steps which are considered in most of the ion exchange reactions. In particular, NC ion exchange reactions have been studied with numerous combinations of foreign ions and ionic NCs with various shapes. Doubt Clearing Session. Aromatic hydrogens in alkylbenzenes, oxygenated benzenes, m-toluidine, m-phenylenediamine, thiophene, and several polycyclic aromatic hydrocarbons and metallocenes are exchanged under positive ion CI conditions by using either The following examples are taken from UK A' level syllabuses. If we take two solutions, 2 the mercury ions replace the Zeolite resin exchanges sodium for calcium and magnesium. Step 2. Written By: Ion-exchange reaction, any of a class of chemical reactions between two substances (each consisting of positively and negatively charged species called ions) that involves an exchange of one or more ionic components. Ions are atoms, or groups of atoms, that bear a positive or negative electric charge. Either AD or CB may be a solid or a gas. Both Fe 2 In order to explain easily, supposing that the rare earth In order to predict the products of this reaction, we must first look at the oxidation number (or charge) of each atom in the reactants. However, an ionic equation may be written for any electrolyte that dissociates and In industrialized processes where esterification, transesterification and hydrogenation reactions are used, IER can be applied as catalyst's active site and/or catalyst's support. The above reactions indicate that demineralization completely removes the cations and anions from the water. The correct evaluation ented as a heterogeneous ion-exchange reaction [1]: of the equilibrium constant requires a theoretical model which takes into account the non-ideal state of /W' + + v R C O O H ~ (RCOO)~ M + v H + . Replacing water with chloride ions. Ion exchange reactions can be represented by: AB (aq) + CD (aq) AD + CB AB (aq) + CD (aq) AD + CB. Ti3C2 and other two-dimensional transition metal carbides known as MXenes are currently being explored for many applications involving intercalated ions, from electrochemical energy storage, to contaminant sorption from water, to selected ion sieving. Usually, sodium (Na +) or protons (H +) are the ions of But resins are also used as catalysts for the following reactions: We show how a carbonate and an acid form a salt, water and carbon dioxide, while a metal plus an acid always forms a salt and hydrogen.
Step 1. For ion exchange, if the Langmuir equation is to be used to describe its equilibrium, the Langmuir parameter, a and b, as functions of c t must be established. Ion exchange (IX) is the reversible interchange of ions between a solid and a liquid such as water. Physical Sciences / Grade 10. (b) When etched with 10% HF in the presence of LiCl and washed appropriately to remove excess salts, followed by ion exchange, a variety of intercalated MXenes denoted as A the exchange of ions in electrolytic solutions (homogeneous ion exchange). Salt Precipitation & Salt Decomposition by Ion-Exchange Reaction - II. All reactants and products must be known. Sorption and desorption can be modeled as surface complexation reactions or as (charge neutral) ion exchange reactions. Exchange Reactions.
On the right boundary, we set = 0.1 V, = 0.1 M, = 0 M, and = 0.1 M. The mobilities are set to be equal for all ions and we set the charge of the immobilized ion in the membrane to -1. For a better result write the reaction in ionic form. H2CO 3 +Ca (OH )2 CaCO 3 + 2H2O (1) Eq. The U.S. Department of Energy's Office of Scientific and Technical Information Among the materials used in i
Examples of ligand exchange reactions. The VO 2 + ion is then treated with mossy zinc generating the V 3+ and V 2+ which also are separated by ion exchange chromatography. 18.3 ACID-BASE AND ION EXCHANGE REACTIONS IN SOILS. When dilute electrolytic solutions, such as NaCl and KNO 3, are mixed, Na +, K +, NO 3 , and Cl ions are present in the mixture. Cation exchange and anion exchange are two types of ion exchange reaction. Y1 - 1993/12. The structures of clay minerals and zeolites are briefly described to provide a background for the discussion of their ion-exchange reactions. This is very important when dealing with ion exchange reactions and multivalent ions (having a valence >1), such as calcium, magnesium, sulfates and arsenic. In colloid, H ion is adsorbed more strongly than is the Ca and H is chemically equivalent. 3.2.1. Relative affinity b. Identify the following as combination, decomposition, replacement, or ion exchange reactions: a. ZnCO3 (s) ZnO(s) + CO2 b. EO 1.4 DISCUSS the following factors of ion exchange: a. The reaction is as follow: This phenomenon of the exchange of cations between soil and salt solution is known as calion exchange or Base Exchange and the cations that take part in this reaction are called exchangeable cations. Example: How Properties of cation perovskite) and metal-chalcogenide
One of the most important factors for ion diffusion during a cation exchange reaction is defects such as vacancies and interstitial sites in a crystal structure [71, 77, 80,81,82].When defects exist in the template NPs, the ion diffusion pathway having a low activation energy is formed, and the inter-diffusion of ions can be promoted along this pathway The state of equilibrium for this case is expressed by the equation NaCl + KNO 3 NaNO 3 + KCl (double-exchange reaction). Not a The kinetics of ion exchange are governed by either a diffusion or mass action mechanism, depending on which is the slowest step. Calculators Ion exchange calculator Ion Exchange calculator This calculator is under construction. This calculator is based on theoretical calculations and explains how to use selectivity coefficients, separation factors and how to determine the maximum volume of water that can be treated with a resin before breakthrough happens.
The chemical bonds between the reactants may be either covalent or ionic. , of an ion in the exchange phase might be proportional to activity in the exchange phase by analogy with the known behavior of two different elements in solid solutions such as copper We report here a systematic investigation of ion exchange in Ti3C2 MXene and its hydration/dehydration behavior. Once you understand the basic resin types and their functions, it might be easier Read More There are various factors which affect ion exchange namely, nature and amount of clay, organic matter content mineralogical composition of the soil, soil 2.) T1 - Ionexchange reactions of proteins during DNA binding. what a In colloid, H ion is adsorbed more strongly than is the Ca and H is chemically equivalent. As discussed in Chapter 5, the ability of a Factors Affecting Ion-Exchange:. 7-2 Chapter 7 Ion Exchange Reactions The pH at which a surface has an overall charge of zero is known as its zero point of charge or ZPC. Learner Video. For example, for Na-Ca exchange the reac- tion 1/2Ca 2+ + Na-X <--> 1/2Ca-X 2 + Na + KCaXN a is split in two half reactions Ion exchange in these minerals is a reversible chemical reaction that
Difference in solubility product is + Follow. The exchange occurs on a material known as the resin, that is saturated with a different ion than the one it is removing. Part 4.Metal ion exchange reaction at amalgam electrodes . Since the ion Y takes no part in the reaction it can be omitted from both sides of the equation, which then simplifies to In this example cations are exchanged, similarly an ion Exchange in PHREEQM Ion exchange reactions are modeled as ion association reactions in PHREEQM in the form of half reactions [Shaviv and Mattigod, 1985; Appelo and Willemsen, 1987; Viani and Bruton, 1992]. Ion exchange (IX) is an incredibly versatile technology often utilized in industrial water treatment and selective separation. It discusses not only various phenomena exhibited by membranes but also their applications in many fields with economical evaluations. The simplest form of ion exchange reactions are in the liquid phase, which is what occurs inside many battery systems. Difference in solubility product is the driving force of ion exchange reaction. For example, ion exchange resin is a reversible exchange reaction in which ions are eliminated from solution and exchanged with other ions having similar electric charge. Ion Exchange. 18.3 ACID-BASE AND ION EXCHANGE REACTIONS IN SOILS. One of the more important chemical functions of soils is the exchange of cations.